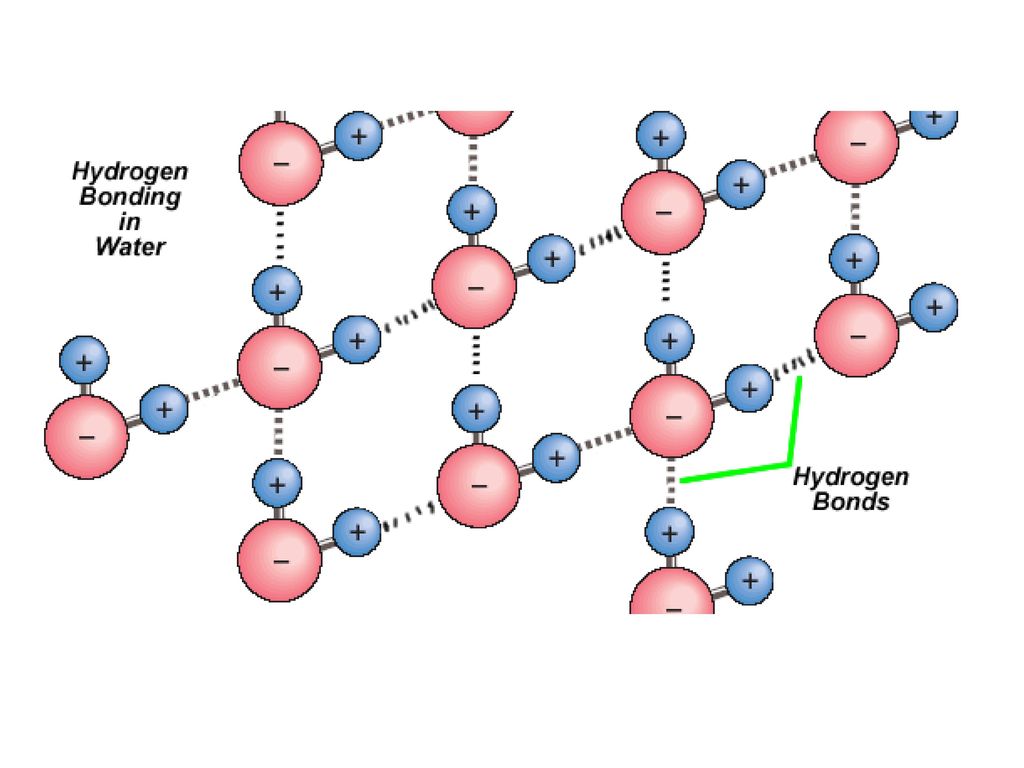
Water consists of polar molecules, they stick to each other and to other surfaces like a magnet.
The oxygen atom is more electronegative than the hydrogen atoms, creating polar bonds. Additionally, the bent shape of the water molecule means that the dipole moments do not cancel each other out, making water an overall polar molecule. This polarity allows water to form hydrogen bonds and is crucial for its many properties, such as its solvent capabilities.
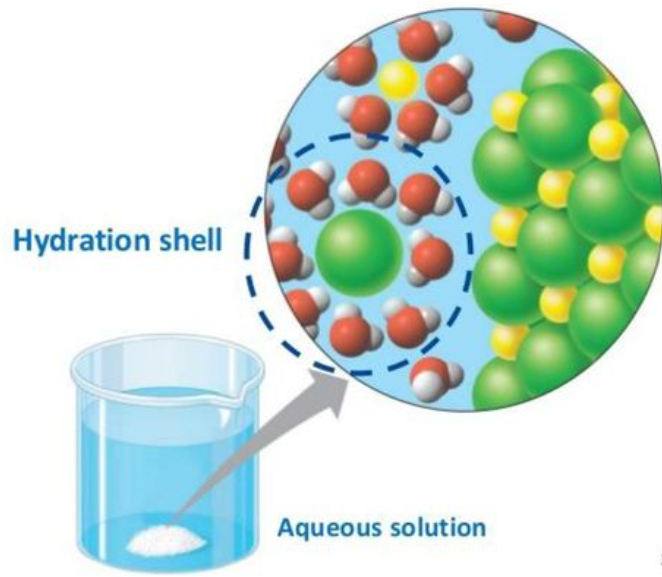
Solvation shells, also known as hydration shells when the solvent is water, are layers of solvent molecules that surround a solute particle when it dissolves. These shells play a crucial role in the process of solvation, which is how a solute becomes dissolved in a solvent. Here’s a detailed look at what they are and how they work:
Formation of Solvation Shells
Interaction with Solvent: When a solute, like a salt, dissolves in a solvent like water, the solvent molecules arrange themselves around the solute. The arrangement depends on the nature of the solute and solvent molecules—particularly their charges and polarities.
Orientation of Solvent Molecules: In the case of water—a polar solvent—the water molecules orient themselves around ions of the dissolved solute in a specific manner. Positive parts of the water molecules (hydrogen atoms) face negatively charged solute ions, and the negative parts (oxygen atoms) face positively charged ions.
Characteristics of Solvation Shells
First Solvation Shell: This is the layer of solvent molecules that is directly in contact with the solute. These solvent molecules are strongly attracted to the solute, often forming specific, stable arrangements. For instance, water molecules around a sodium ion (Na+) in an aqueous solution might form a specific geometric arrangement based on their interactions.
Second and Subsequent Shells: Beyond the first layer, there can be one or more additional layers of solvent molecules. These layers are increasingly less organized compared to the first solvation shell, as the direct influence of the solute diminishes. These solvent molecules are more influenced by their interactions with other solvent molecules rather than with the solute.
Role of Solvation Shells
Stabilization of Ions: Solvation helps stabilize ions in solution by surrounding them with solvent molecules, reducing their potential to recombine into undissolved salt.
Influence on Solubility: The strength and structure of the solvation shells can influence the solubility of a substance. Strong solvation can help keep a solute dissolved and can affect the saturation point of the solution.
Impact on Chemical Reactions: The presence and nature of solvation shells can also influence the reactivity of solute particles. In biochemical contexts, for example, the structure and dynamics of hydration shells around enzymes and substrates can significantly affect reaction rates and mechanisms.
Solvation shells are thus essential for understanding many fundamental and practical aspects of solution chemistry, influencing everything from the behavior of electrolytes in batteries to the function of biological molecules in cellular environments.
The reduction of water molecule clusters through the increased equilibrium in hydration shells is a complex process involving the interactions between water molecules and solute ions. This process fundamentally alters the dynamics and structure of water in the solution. Here’s an in-depth look at how increasing equilibrium in hydration shells leads to the reduction of water molecule clusters:
Understanding Water Molecule Clusters
Water molecules naturally form clusters due to hydrogen bonding, where the hydrogen atom of one water molecule forms a bond with the oxygen atom of another. These clusters can be quite dynamic, constantly forming and breaking apart.
Role of Hydration Shells
When a solute (like salt) is introduced into water, the solute ions disrupt the hydrogen bonding among water molecules. This is because the water molecules reorient themselves to stabilize the ions:
Formation of Hydration Shells: Water molecules surround the solute ions, forming structured layers known as hydration shells. The first shell directly interacts with the ion, while subsequent shells have less direct interaction.
Impact on Water Clusters: The formation of these hydration shells disrupts the existing network of hydrogen bonds among water molecules. The structured arrangement around the ions prevents these water molecules from participating in the larger, natural clustering behavior typical in pure water.
Increasing Equilibrium in Hydration Shells
Stabilization of Ions: As the hydration shells stabilize, there is a reduction in the free energy of the system. This stabilization comes from the optimal arrangement of water molecules around each ion, minimizing repulsive forces and maximizing attractive interactions.
Enhanced Solvation: With increased equilibrium, the solvation process becomes more efficient. This efficiency is characterized by a more stable and orderly arrangement of water molecules in the hydration shells. The more ordered these shells are, the less likely water molecules are to form large, disordered clusters.
Reduction of Water Clusters: As the hydration shells stabilize, the overall water structure in the solution changes. The tightly bound water molecules in the shells are less likely to join freely with other water molecules, thus reducing the size and frequency of water molecule clusters in the solution.
Practical Implications
This reduction in clustering has several implications, especially in biochemical and industrial processes:
Increased Solubility: Improved solvation can increase the solubility of solutes, as water molecules are more effectively used to stabilize ions rather than forming clusters.
Altered Physical Properties: Solutions where water clustering is reduced may exhibit different physical properties, such as viscosity and heat capacity, compared to pure water.
Biochemical Reactions: In biological systems, the restructuring of water around macromolecules like proteins can affect their stability, folding, and interactions, crucial for processes like enzyme activity and substrate binding.
Overall, the reduction of water molecule clusters by increasing equilibrium in hydration shells can significantly influence the behavior of solutions and the interactions within them. This understanding is critical for fields ranging from chemistry and material science to biology and medicine.
